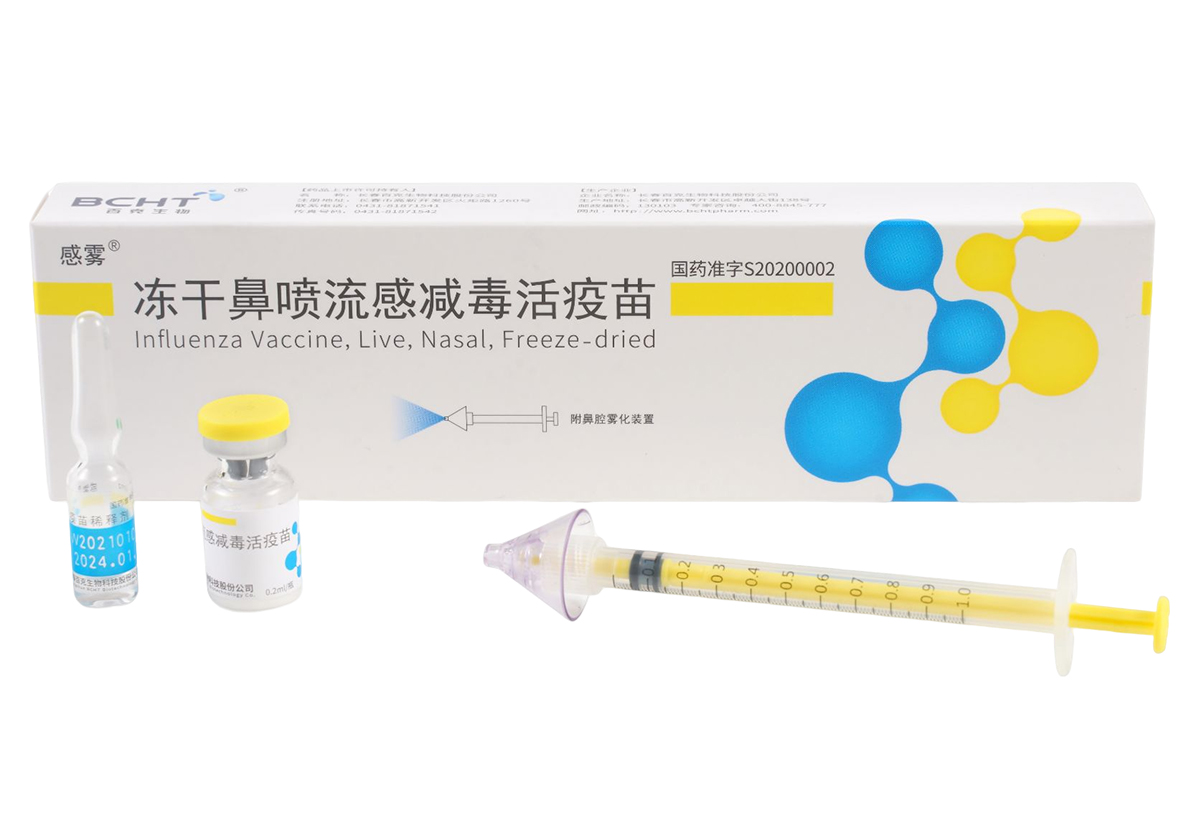
“Freeze-dried nasal spray live attenuated influenza vaccine” by BCHT is opening doors to a new era of influenza prevention in China. As the country's sole provider of this innovative product, BCHT has gained international recog nition by actively contributing to the World Health Organization's (WHO) Global Influenza Action Plan (GAP) initia tive. With millions of doses administered worldwide, this live attenuated influenza vaccine has emerged as a powerful tool in the fight against influenza.
Designed for individuals aged 3 to 17, this groundbreaking vaccine simplifies the vaccination process by requiring only one annual administration. By triggering the body's immune response against the influenza virus, the vaccine significantly reduces the risk of contraction from vaccine-related types of influenza.